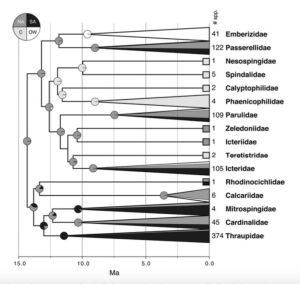
UNM Ornithologists read about 100 papers together each year. About 1/3 of these are discussed formally in our Journal Club, in which 10-15 participants dissect and critique them. The other papers include joint manuscript reviews, papers that were nominated but not discussed formally, or papers we read for collaborative research projects. Some are high-impact papers, some are New Mexico related, and some are interesting only for highly specific reasons known only to us. I list my ten favorites of 2018 here, unranked; these are the ones that best withstood the criticism of our group. Below the top ten you can find the other papers listed alphabetically.
Our paper choices are somewhat arbitrary cannot be comprehensive for any of the subdisciplines represented. If your favorite paper is not included, link to it in the comments section. The brief commentaries under selected papers are exclusively mine, may be inaccurate or unfair, and might not represent the sentiment of the group. Thanks to these core Journal Club participants for 2018: Mike Andersen, Chris Anderson, Lisa Barrow, Selina Bauernfeind, Serina Brady, Malé Castro, Paxton Cruz, Chauncey Gadek, Levi Gray, Tina Guo, Ethan Gyllenhaal, Andy Johnson, Xena Mapel, Peter Mattison, Jenna McCullough, Moses Michelsohn, Kristen Oliver, Oona Takano, Bill Talbot, Britt White, Dani Wiley, and Jessie Williamson.
Ten favorites from 2018 (unranked):
- Antonelli, A., Zizka, A., Carvalho, F. A., Scharn, R., Bacon, C. D., Silvestro, D., & Condamine, F. L. (2018). Amazonia is the primary source of Neotropical biodiversity. Proceedings of the National Academy of Sciences, 115(23), 6034–6039. http://doi.org/10.1073/pnas.1713819115
- The 508-page supplement gives a good indication of the underlying substance of this massive synthetic work. The main conclusion (see title) is important; our group noted that it may have been heavily influenced by the much higher diversity in the Amazon region than elsewhere, plus the fact that ‘Amazonia’ was defined here to include the Guyana Shield, Chocó, Darien, and Andean forests up to treeline. The most interesting part of the paper, IMHO, were the timelines of ‘normalized’ rates of exchange between regions for each of six vertebrate taxa. Those are truly fascinating and deserve further consideration.
- Barrera-Guzmán, A. O., Aleixo, A., Shawkey, M. D., & Weir, J. T. (2018). Hybrid speciation leads to novel male secondary sexual ornamentation of an Amazonian bird. Proceedings of the National Academy of Sciences of the United States of America, 115(2), E218–E225. http://doi.org/10.1073/pnas.1717319115
- We loved this paper. The one critique that couldn’t be resolved in our discussion was why the authors did not apply the coalescent models to test more possible scenarios of speciation and gene flow. We concluded that the case for hybrid speciation and the case for the mechanistic link between hybridization and plumage signals were short of 100% definitive, but it was a beautiful paper, nonetheless. I consider it a ‘must read’.
- Bay, R. A., Harrigan, R. J., Underwood, V. L., Gibbs, H. L., Smith, T. B., & Ruegg, K. (2018). Genomic signals of selection predict climate-driven population declines in a migratory bird. Science, 359(6371), 83–86. http://doi.org/10.1126/science.aan4380
- This is groundbreaking work, and it’s also potentially pushing the limits of genomic inference: see the Technical Comment and response here: http://doi.org/10.1126/science.aat7279
- Clark, N. J. (2018). Phylogenetic uniqueness, not latitude, explains the diversity of avian blood parasite communities worldwide. Global Ecology and Biogeography, 27(6), 744–755. http://doi.org/10.1111/geb.12741
- Clark is doing fantastic faunal analyses on haemosporidians, of which this is just one example.
- Freeman, B. G., Scholer, M. N., Ruiz-Gutierrez, V., & Fitzpatrick, J. W. (2018). Climate change causes upslope shifts and mountaintop extirpations in a tropical bird community. Proceedings of the National Academy of Sciences, 115(47), 11982–11987. http://doi.org/10.1073/pnas.1804224115
- This is awesome — a rare glimpse into avifaunal change in one of the most diverse but rapidly changing places on earth.
- Irwin, D. E., Milá, B., Toews, D. P. L., Brelsford, A., Kenyon, H. L., Porter, A. N., et al. (2018). A comparison of genomic islands of differentiation across three young avian species pairs. Molecular Ecology, 27(23), 4839–4855. http://doi.org/10.1111/mec.14858
- Thorough description of genomic divergence in these three cases reveals some fundamental aspects of speciation.
- Jones, M. R., Mills, L. S., Alves, P. C., Callahan, C. M., Alves, J. M., Lafferty, D. J. R., et al. (2018). Adaptive introgression underlies polymorphic seasonal camouflage in snowshoe hares. Science, 360(6395), 1355–1358. http://doi.org/10.1126/science.aar5273
- An elegant paper demonstrating decisively how, when, and why a gene for seasonal polymorphism jumped species boundaries from a jackrabbit to a snowshoe hare.
- Morales, H. E., Pavlova, A., Amos, N., Major, R., Kilian, A., Greening, C., & Sunnucks, P. (2018). Concordant divergence of mitogenomes and a mitonuclear gene cluster in bird lineages inhabiting different climates. Nature Ecology & Evolution, 2(8), 1258–1267. http://doi.org/10.1038/s41559-018-0606-3
- Fantastic description of genetic divergence between inland and coastal forms along the east coast of Australia, highly suggestive of mitonuclear coevolution. There were mtDNA and nuclear DNA clines from coastal to inland, across a temperature gradient. mtDNA and some nuclear loci were locally adapted and segregating, mtDNA divergence was high, indicating a deep history of cold adapted and warm adapted genotypes, respectively. My biggest complaint is that one had to read to the 2nd page of the paper just to find out what species was being studied (Eastern Yellow Robin) — are they embarrassed of their study species? Do species even matter any more? Do we go to the field just to study concepts in order to please our journal-editor overlords?!
- Lamichhaney, S., Han, F., Webster, M. T., Andersson, L., Grant, B. R., & Grant, P. R. (2018). Rapid hybrid speciation in Darwin’s finches. Science, 359(6372), 224–228. http://doi.org/10.1126/science.aao4593.
- Fascinating case study and phenomenal dataset.
- Quintero, I., & Jetz, W. (2018). Global elevational diversity and diversification of birds. Nature, 555(7695), 246–250. http://doi.org/10.1038/nature25794
- A super impressive assemblage of avifaunal data and analyses, revealing some fundamental truths about diversification and diversity of birds in mountains.
The rest of our 2018 selections (alphabetical order by 1st author):
- Alcala, N., Jenkins, T., Christe, P., & Vuilleumier, S. (2017). Host shift and cospeciation rate estimation from co-phylogenies. Ecology Letters, 20(8), 1014–1024. http://doi.org/10.1111/ele.12799
- Very welcomed framework for analyzing coevolution of parasites and hosts, even when there’s lots of host-switching, as occurs in haemosporidians.
- Anticona, S. J., Olmos, E. F., Parent, J. R., Rutti, D. M., Velasco, B. A., & Hoese, W. J. (2013). Greater Roadrunner (Geococcyx californianus) Kills Juvenile Desert Cottontail (Sylvilagus audubonii). The Southwestern Naturalist, 58(1), 124–126. http://doi.org/10.1894/0038-4909-58.1.124
- The title says it all — we like roadrunner natural history here in New Mexico.
- Antonelli, A., Ariza, M., Albert, J., Andermann, T., Azevedo, J., Bacon, C., et al. (2018a). Conceptual and empirical advances in Neotropical biodiversity research. PeerJ, 6(5), e5644–53. http://doi.org/10.7717/peerj.5644
- A really nice and comprehensive review with appealing graphics.
- Antonelli, A., Kissling, W. D., Flantua, S. G. A., Bermúdez, M. A., Mulch, A., Muellner-Riehl, A. N., et al. (2018b). Geological and climatic influences on mountain biodiversity. Nature Geoscience, 1–10. http://doi.org/10.1038/s41561-018-0236-z
- Biologists and geologists working together? Awesome.
- Battey, C. J., 2018. Time Lags and Niche Shifts in a Biological Invasion of Hummingbirds. Biorxiv.org. http://doi.org/10.1101/329615
- The expansion of the Anna’s Hummingbird since the early-mid 20th century was a story that needed telling — this paper does so with nice graphics.
- Battey, C. J., & Klicka, J. (2017). Cryptic speciation and gene flow in a migratory songbird Species Complex: Insights from the Red-Eyed Vireo (Vireo olivaceus). Molecular Phylogenetics and Evolution, 113, 67–75. http://doi.org/10.1016/j.ympev.2017.05.006
- Beckman, E. J., Benham, P. M., Cheviron, Z. A., & Witt, C. C. (2018). Detecting introgression despite phylogenetic uncertainty: The case of the South American siskins. Molecular Ecology, 27(22), 4350–4367. http://doi.org/10.1111/mec.14795
- OK, this is one of our own, but we’re proud of it. See also the commentary by James Pease: https://doi.org/10.1111/mec.14851
- Betts, A., Gray, C., Zelek, M., MacLean, R. C., & King, K. C. (2018). High parasite diversity accelerates host adaptation and diversification. Science, 360(6391), 907–911. http://doi.org/10.1126/science.aam9974
- Before you get too excited… this was in a test tube, not in the wild! Nonetheless, it’s a great paper and very thought-provoking.
- Birkhead, T. R., Thompson, J. E., Biggins, J. D., & Montgomerie, R. (2018). The evolution of egg shape in birds: selection during the incubation period. Ibis, 55, 105–14. http://doi.org/10.1111/ibi.12658
- This is more-or-less a rebuttal to the Stoddard et al. paper in Science the other year. Both are worth reading.
- Boast, A. P., Weyrich, L. S., Wood, J. R., Metcalf, J. L., Knight, R., & Cooper, A. (2018). Coprolites reveal ecological interactions lost with the extinction of New Zealand birds. Proceedings of the National Academy of Sciences, 115(7), 1546–1551. http://doi.org/10.1073/pnas.1712337115
- Bruxaux, J., Gabrielli, M., Ashari, H., Prŷs-Jones, R., Joseph, L., Milá, B., et al. (2017). Recovering the evolutionary history of crowned pigeons (Columbidae_ Goura)_ Implications for the biogeography and conservation of New Guinean lowland birds. Molecular Phylogenetics and Evolution, 120, 248–258. http://doi.org/10.1016/j.ympev.2017.11.022
- Budischak, S. A., Wiria, A. E., Hamid, F., Wammes, L. J., Kaisar, M. M. M., van Lieshout, L., et al. (2018). Competing for blood: the ecology of parasite resource competition in human malaria-helminth co-infections. Ecology Letters, 21(4), 536–545. http://doi.org/10.1111/ele.12919
- Campbell-Staton, S. C., Bare, A., Losos, J. B., Edwards, S. V., & Cheviron, Z. A. (2018). Physiological and regulatory underpinnings of geographic variation in reptilian cold tolerance across a latitudinal cline. Molecular Ecology, 27(9), 2243–2255. http://doi.org/10.1111/mec.14580
- Carstens, B. C., Morales, A. E., Field, K., & Pelletier, T. A. (2018). A global analysis of bats using automated comparative phylogeography uncovers a surprising impact of Pleistocene glaciation. Journal of Biogeography, 45(8), 1795–1805. http://doi.org/10.1111/jbi.13382
- This paper presented an analysis of 100’s of bat mtdna phylogeography datasets to test for expansion or bottlenecks after LGM. The results were mostly indecisive, with only a handful of datasets supporting either scenario with confidence. Neotropical species tended to have been subject to bottlenecks. The automated data harvesting method revealed weaknesses with Genbank and GBIF datasets. Natural selection effects were ignored (which may be problematic, see Bazin et al. 2006, http://science.sciencemag.org/content/312/5773/570). Some of our group had numerous issues with the paper, some defended it, and the discussion was rollicking.
- Chan, W.-P., Chen, I.-C., Colwell, R. K., Liu, W.-C., Huang, C.-Y., & Shen, S.-F. (2018). Response to Qian et al. (2017): Daily and seasonal climate variations are both critical in the evolution of species’ elevational range size. Journal of Biogeography, 45(12), 2832–2836. http://doi.org/10.1111/jbi.13449
- Chase, J. M., McGill, B. J., McGlinn, D. J., May, F., Blowes, S. A., Xiao, X., et al. (2018). Embracing scale-dependence to achieve a deeper understanding of biodiversity and its change across communities. Ecology Letters, 21(11), 1737–1751. http://doi.org/10.1111/ele.13151
- Chen, N., Cosgrove, E. J., Bowman, R., Fitzpatrick, J. W., & Clark, A. G. (2016). Genomic Consequences of Population Decline in the Endangered Florida Scrub-Jay. Current Biology, 26(21), 2974–2979. http://doi.org/10.1016/j.cub.2016.08.062
- Cheviron, Z. A., & Swanson, D. L. (2017). Comparative Transcriptomics of Seasonal Phenotypic Flexibility in Two North American Songbirds. Integrative and Comparative Biology, 57(5), 1040–1054. http://doi.org/10.1093/icb/icx118
- This is a groundbreaking paper, simple in design (two species X two seasons), but really novel in its rigorous application of transcriptomics to bird seasonal adjustments. There were similar seasonal gene-expression shifts in chickadees and goldfinches overall, with some species specific patterns too. Some imperfect venn diagrams (paging Scott Walker!), but a really interesting and informative paper regarding seasonal gene expression shifts.
- Corti, M., Podofillini, S., Griggio, M., Gianfranceschi, L., Ducrest, A.-L., Roulin, A., et al. (2018). Sequence variation in melanocortin-1-receptor and tyrosinase-related protein 1 genes and their relationship with melanin-based plumage trait expression in Lesser Kestrel (Falco naumanni) males. Journal of Ornithology, 1–6. http://doi.org/10.1007/s10336-018-1537-0
-
Divis, P. C. S., Duffy, C. W., Kadir, K. A., Singh, B., & Conway, D. J. (2018). Genome-wide mosaicism in divergence between zoonotic malaria parasite subpopulations with separate sympatric transmission cycles. Molecular Ecology, 27(4), 860–870. http://doi.org/10.1111/mec.14477. Human malaria studies get better data, so they provide useful models for early-stage divergence in avian malaria.
- Drury, J. P., Tobias, J. A., Burns, K. J., Mason, N. A., Shultz, A. J., & Morlon, H. (2018). Contrasting impacts of competition on ecological and social trait evolution in songbirds. PLOS Biology, 16(1), e2003563–23. http://doi.org/10.1371/journal.pbio.2003563
- This probably goes a little too far — inferring competition based on distribution and phylogenetic patterns alone is still fraught. Nonetheless, the analyses and ideas are interesting in their own right.
- Ellis, V. A., & Bensch, S. (2018). Host specificity of avian haemosporidian parasites is unrelated among sister lineages but shows phylogenetic signal across larger clades. International Journal for Parasitology, 1–6. http://doi.org/10.1016/j.ijpara.2018.05.005
- Fecchio, A., Bell, J. A., Collins, M. D., Farias, I. P., Trisos, C. H., Tobias, J. A., Tkach, V. V., Weckstein, J. D., Ricklefs, R. E., & Batalha-Filho, H. (2018a). Diversification by host switching and dispersal shaped the diversity and distribution of avian malaria parasites in Amazonia. Oikos, 127(9), 1233–1242. http://doi.org/10.1111/oik.05115
- Felice, R. N., & Goswami, A. (2018). Developmental origins of mosaic evolution in the avian cranium. Proceedings of the National Academy of Sciences of the United States of America, 115(3), 555–560. http://doi.org/10.1073/pnas.1716437115
- Not really surprising that the face (i.e. beak) is under a lot of selection. Back of head not so much. Also, if you atomize into parts, you’ll find mosaic evolution. But the paper was nifty anyway.
- Field, R., & Qian, H. (2018). No empirical evidence to support the hypothesis that daily climate variation has an effect on species’ elevational range size: Reply to Chan et al. Journal of Biogeography, 45(12), 2827–2832. http://doi.org/10.1111/jbi.13372
- Fitt, R. N. L., Palmer, S., Hand, C., Travis, J. M. J., & Lancaster, L. T. (2018). Towards an interactive, process-based approach to understanding range shifts: developmental and environmental dependencies matter. Ecography, 147, 381–24. http://doi.org/10.1111/ecog.03975
- Funk, V. A. (2018). Collections-based science in the 21st Century. Journal of Systematics and Evolution, 56(3), 175–193. http://doi.org/10.1111/jse.12315
- Gallo, S. S. M., Ederli, N. B., & Oliveira, F. C. R. (2017). Hematological and morphometric differences of blood cells from rheas, Rhea americana (Struthioniformes: Rheidae) on two conservation farms. Brazilian Journal of Biology, 77(2), 227–233. http://doi.org/10.1590/1519-6984.07915
- George, R. J., Plog, S., Watson, A. S., Schmidt, K. L., Culleton, B. J., Harper, T. K., et al. (2018). Archaeogenomic evidence from the southwestern US points to a pre-Hispanic scarlet macaw breeding colony. Proceedings of the National Academy of Sciences, 115(35), 8740–8745. http://doi.org/10.1073/pnas.1805856115
- Gilbert, K. J., Peischl, S., & Excoffier, L. (2018). Mutation load dynamics during environmentally-driven range shifts. PLoS Genetics, 14(9), e1007450–14. http://doi.org/10.1371/journal.pgen.1007450
- Glassman, S. I., Wang, I. J., & Bruns, T. D. (2017). Environmental filtering by pH and soil nutrients drives community assembly in fungi at fine spatial scales. Molecular Ecology, 26(24), 6960–6973. http://doi.org/10.1111/mec.14414
- Gómez-Sánchez, D., Olalde, I., Sastre, N., Enseñat, C., Carrasco, R., Marques-Bonet, T., et al. (2018). On the path to extinction: Inbreeding and admixture in a declining grey wolf population. Molecular Ecology, 27(18), 3599–3612. http://doi.org/10.1111/mec.14824
- Grant, P. R., & Grant, B. R. (2018). Role of sexual imprinting in assortative mating and premating isolation in Darwin’s finches. Proceedings of the National Academy of Sciences, 115(46), E10879–E10887. http://doi.org/10.1073/pnas.1813662115
- Halbritter, A. H., Fior, S., Keller, I., Billeter, R., Edwards, P. J., Holderegger, R., et al. (2018). Trait differentiation and adaptation of plants along elevation gradients. Journal of Evolutionary Biology, 31(6), 784–800. http://doi.org/10.1111/jeb.13262
- Haupaix, N., Curantz, C., Bailleul, R., Beck, S., Robic, A., & Manceau, M. (2018). The periodic coloration in birds forms through a prepattern of somite origin. Science, 361(6408), eaar4777–8. http://doi.org/10.1126/science.aar4777
- Hazzi, N. A., Moreno, J. S., Ortiz-Movliav, C., & Palacio, R. D. (2018). Biogeographic regions and events of isolation and diversification of the endemic biota of the tropical Andes. Proceedings of the National Academy of Sciences, 115(31), 7985–7990. http://doi.org/10.1073/pnas.1803908115
- This synthesis paper only analyzed 14 small clades, ignoring many recent phylogenies. Misspellings of bird names were also an issue
- This synthesis paper only analyzed 14 small clades, ignoring many recent phylogenies. Misspellings of bird names were also an issue
- Hoffmann, F. G., Vandewege, M. W., Storz, J. F., & Opazo, J. C. (2018). Gene Turnover and Diversification of the α- and β-Globin Gene Families in Sauropsid Vertebrates. Genome Biology and Evolution, 10(1), 344–358. http://doi.org/10.1093/gbe/evy001
- Holt, R. D., & Bonsall, M. B. (2017). Apparent Competition. Annual Review of Ecology, Evolution, and Systematics, 48(1), 447–471. http://doi.org/10.1146/annurev-ecolsys-110316-022628
- Huang, X., Ellis, V. A., Jönsson, J., & Bensch, S. (2018). Generalist haemosporidian parasites are better adapted to a subset of host species in a multiple host community. Molecular Ecology, 27(21), 4336–4346. http://doi.org/10.1111/mec.14856
- Jax, E., Wink, M., & Kraus, R. H. S. (2018). Avian transcriptomics: opportunities and challenges. Journal of Ornithology, 159(3), 599–629. http://doi.org/10.1007/s10336-018-1532-5
- Judson, O. P. (2017). The energy expansions of evolution. Nature Ecology & Evolution, 1(6), 0138–10. http://doi.org/10.1038/s41559-017-0138
- Kearns, A. M., Restani, M., Szabo, I., Schrøder-Nielsen, A., Kim, J. A., Richardson, H. M., et al. (2018). Genomic evidence of speciation reversal in ravens. Nature Communications, 1–13. http://doi.org/10.1038/s41467-018-03294-w
- “Speciation reversal” is a tough sell here, but a fascinating case study anyway.
- Kingsolver, J. G., & Buckley, L. B. (2017). Evolution of plasticity and adaptive responses to climate change along climate gradients. Proceedings of the Royal Society of London B: Biological Sciences, 284(1860), 20170386–7. http://doi.org/10.1098/rspb.2017.0386
- Londoño, G. A., Chappell, M. A., Jankowski, J. E., & Robinson, S. K. (2016). Do thermoregulatory costs limit altitude distributions of Andean forest birds? Functional Ecology, 31(1), 204–215. http://doi.org/10.1111/1365-2435.12697
- Manzoli, D. O. E., Saravia-Pietropaolo, M. J., Antoniazzi, L. R., Barengo, E., Arce, S. I., Quiroga, M. A., & Beldomenico, P. M. (2018). Contrasting consequences of different defence strategies in a natural multihost-parasite system. International Journal for Parasitology, 48(6), 445–455. http://doi.org/10.1016/j.ijpara.2017.11.001
- Same parasite has really different effects on different host species in the same communities, and this paper disentangles tolerance and resistance to show how different defense strategies underlie the different outcomes.
- Miles, M. C., Goller, F., eLife, M. F., 2018. Physiological constraint on acrobatic courtship behavior underlies rapid sympatric speciation in bearded manakins. Elife. http://doi.org/10.7554/eLife.40630.001
- The sympatric speciation part is not the strongest part of this paper, but it’s worth reading.
- Mueller, J. C., Kuhl, H., Boerno, S., Tella, J. L., Carrete, M., & Kempenaers, B. (2018). Evolution of genomic variation in the burrowing owl in response to recent colonization of urban areas. Proceedings of the Royal Society of London B: Biological Sciences, 285(1878), 20180206–9. http://doi.org/10.1098/rspb.2018.0206
- Great to see this Mueller Report issued in a timely fashion.
- Mueller, N. F., Ogilvie, H., Zhang, C. (2018). Inference of species histories in the presence of gene flow. Biorxiv.org. http://doi.org/10.1101/348391
- Naka, L. N., & Brumfield, R. T. (2018). The dual role of Amazonian rivers in the generation and maintenance of avian diversity. Science Advances, 4(8), eaar8575. http://doi.org/10.1126/sciadv.aar8575
- First off, this is an excellent paper with a sizable, original empirical dataset. And it’s beautifully written. The “dual roles” of rivers were defined as (1) primary divergence and (2) maintenance of separation; but it was never easy to distinguish one from the other. Divergences were mostly recent, with fewer old ones, and were broadly consistent with neutral process of diversification. Interesting contrast with our “dual roles of Andean topography” paper, in which the dual roles were barriers and divergent selection pressures (https://bmcevolbiol.biomedcentral.com/articles/10.1186/s12862-016-0595-2). Naturally, I prefer the latter framework 😉
- Ng, J. W., Knight, E. C., Scarpignato, A. L., Harrison, A. L., Bayne, E. M., & Marra, P. P. (2018). First full annual cycle tracking of a declining aerial insectivorous bird, the Common Nighthawk ( Chordeiles minor), identifies migration routes, nonbreeding habitat, and breeding site fidelity. Canadian Journal of Zoology, 96(8), 869–875. http://doi.org/10.1139/cjz-2017-0098
- This is just so cool. Nighthawks rock.
- Pacheco, M. A. N., Cepeda, A. S., Bernotienė, R., Lotta, I. A., Matta, N. E., Valkiūnas, G., & Escalante, A. A. (2018a). Primers targeting mitochondrial genes of avian haemosporidians: PCR detection and differential DNA amplification of parasites belonging to different genera. International Journal for Parasitology, 48(8), 657–670. http://doi.org/10.1016/j.ijpara.2018.02.003
- A tool we’ll use.
- Pan, S., Zhang, T., Rong, Z., Hu, L., Gu, Z., Wu, Q., et al. (2017). Population transcriptomes reveal synergistic responses of DNA polymorphism and RNA expression to extreme environments on the Qinghai-Tibetan Plateau in a predatory bird. Molecular Ecology, 26(11), 2993–3010. http://doi.org/10.1111/mec.14090
- EPAS1! We liked this study overall. Tip 1: if you do a single-species study, put the name of the species in the title (see also Morales et al. 2018, above). Tip 2: if you studied a bird as cool as the Saker Falcon, put the name of the species in the title!
- Patricelli, G. L., Hebets, E. A., & Mendelson, T. C. (2018). Book review of Prum, R. O. 2018. The evolution of beauty: How Darwin’s forgotten theory of mate choice shapes the animal world-and us (2017), Doubleday, 428 pages, ISBN: 9780385537216. Evolution, 323, 152–10. http://doi.org/10.1111/evo.13629
- This is a must-read for anyone interested in both sex and controversy.
- Paxton, E. H., Camp, R. J., Gorresen, P. M., Crampton, L. H., Leonard, D. L., Jr., & VanderWerf, E. A. (2016). Collapsing avian community on a Hawaiian island. Science Advances, 2(9), e1600029–9. http://doi.org/10.1126/sciadv.1600029
- Depressing.
- Penn, J. L., Deutsch, C., Payne, J. L., & Sperling, E. A. (2018). Temperature-dependent hypoxia explains biogeography and severity of end-Permian marine mass extinction. Science, 362(6419), eaat1327–8. http://doi.org/10.1126/science.aat1327
- Phillips, A. G., Töpfer, T., Rahbek, C., Böhning-Gaese, K., & Fritz, S. A. (2018). Effects of phylogeny and geography on ecomorphological traits in passerine bird clades. Journal of Biogeography, 45(10), 2337–2347. http://doi.org/10.1111/jbi.13383
- Pulido-Santacruz, P., Aleixo, A., & Weir, J. T. (2018). Morphologically cryptic Amazonian bird species pairs exhibit strong postzygotic reproductive isolation. Proceedings of the Royal Society of London B: Biological Sciences, 285(1874), 20172081–9. http://doi.org/10.1098/rspb.2017.2081
- Pulgarín, P., Gómez, C., Bayly, N. J., Bensch, S., FitzGerald, A. M., Starkloff, N., et al. (2018a). Migratory birds as vehicles for parasite dispersal? Infection by avian haemosporidians over the year and throughout the range of a long‐distance migrant. Journal of Biogeography, 331, 296. http://doi.org/10.1111/jbi.13453
- Pulgarín, P., Gómez, J. P., Robinson, S., Ricklefs, R. E., & Cadena, C. D. (2018b). Host species, and not environment, predicts variation in blood parasite prevalence, distribution, and diversity along a humidity gradient in northern South America. Ecology and Evolution, 8(8), 3800–3814. http://doi.org/10.1002/ece3.3785
- Ramos, J. S. L., Delmore, K. E., & Liedvogel, M. (2017). Candidate genes for migration do not distinguish migratory and non-migratory birds. Journal of Comparative Physiology A, 203(6), 383–397. http://doi.org/10.1007/s00359-017-1184-6
- Rangel, T. F., Edwards, N. R., Holden, P. B., Diniz-Filho, J. A. F., Gosling, W. D., Coelho, M. T. P., et al. (2018). Modeling the ecology and evolution of biodiversity: Biogeographical cradles, museums, and graves. Science, 361(6399), eaar5452–15. http://doi.org/10.1126/science.aar5452
- Still coming to grips with this impressive modeling effort and what it means.
- Ribas, C. C., Aleixo, A., Gubili, C., d’Horta, F. M., Brumfield, R. T., & Cracraft, J. (2018). Biogeography and diversification of Rhegmatorhina (Aves: Thamnophilidae): Implications for the evolution of Amazonian landscapes during the Quaternary. Journal of Biogeography, 45(4), 917–928. http://doi.org/10.1111/jbi.13169
- Ricklefs, R. E., Ellis, V. A., Medeiros, M. C., & Svensson-Coelho, M. (2018). Duration of embryo development and the prevalence of haematozoan blood parasites in birds. The Auk, 135(2), 276–283. http://doi.org/10.1642/AUK-17-123.1
- Roesti, M. (2018). Varied Genomic Responses to Maladaptive Gene Flow and Their Evidence. Genes, 9(6), 298–16. http://doi.org/10.3390/genes9060298
- Ruegg, K., Bay, R. A., Anderson, E. C., Saracco, J. F., Harrigan, R. J., Whitfield, M., et al. (2018). Ecological genomics predicts climate vulnerability in an endangered southwestern songbird. Ecology Letters, 21(7), 1085–1096. http://doi.org/10.1111/ele.12977
- See also Bay et al., above.
- Sackton, T. B., Grayson, P., Cloutier, A., Hu, Z., Liu, J. S., Wheeler, N. E., et al. (2018). Convergent regulatory evolution and the origin of flightlessness in palaeognathous birds, 1–31. Biorxiv. http://doi.org/10.1101/262584
- Santos, J. C., Tarvin, R. D., O’Connell, L. A., Blackburn, D. C., & Coloma, L. A. (2018). Diversity within diversity: Parasite species richness in poison frogs assessed by transcriptomics. Molecular Phylogenetics and Evolution, 125, 40–50. http://doi.org/10.1016/j.ympev.2018.03.015
- Nifty, yes, but ultimately this method has a ways to go.
- Schindel, D. E., & Cook, J. A. (2018). The next generation of natural history collections. PLOS Biology, 16(7), e2006125–8. http://doi.org/10.1371/journal.pbio.2006125
- Schmitt, C. J., Cook, J. A., Zamudio, K. R., & Edwards, S. V. (2019). Museum specimens of terrestrial vertebrates are sensitive indicators of environmental change in the Anthropocene. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 374(1763), 20170387–10. http://doi.org/10.1098/rstb.2017.0387
- Schweizer, M., Warmuth, V., Alaei Kakhki, N., Aliabadian, M., Förschler, M., Shirihai, H., et al. (2018). Parallel plumage colour evolution and introgressive hybridization in wheatears. Journal of Evolutionary Biology, 26, 229–12. http://doi.org/10.1111/jeb.13401
- Scridel, D., Brambilla, M., Martin, K., Lehikoinen, A., Iemma, A., Matteo, A., et al. (2018). A review and meta-analysis of the effects of climate change on Holarctic mountain and upland bird populations. Ibis, 160(3), 489–515. http://doi.org/10.1111/ibi.12585
- Seeholzer, G. F., & Brumfield, R. T. (2017). Isolation by distance, not incipient ecological speciation, explains genetic differentiation in an Andean songbird (Aves: Furnariidae: Cranioleuca antisiensis, Line-cheeked Spinetail) despite near threefold body size change across an environmental gradient. Molecular Ecology, 27(1), 279–296. http://doi.org/10.1111/mec.14429
- This is a great paper to help us understand ecogeographic variation in Andean birds.
- Sheldon, K. S., Huey, R. B., Kaspari, M., & Sanders, N. J. (2018). Fifty Years of Mountain Passes: A Perspective on Dan Janzen’s Classic Article. American Naturalist, 191(5), 553–565. http://doi.org/10.1086/697046
- Sjöberg, S., Pedersen, L., Malmiga, G., Alerstam, T., Hansson, B., HASSELQUIST, D., et al. (2018). Barometer logging reveals new dimensions of individual songbird migration. Journal of Avian Biology, 49(9), e01821–9. http://doi.org/10.1111/jav.01821
- Smith, C. C. R., Flaxman, S. M., Scordato, E. S. C., Kane, N. C., Hund, A. K., Sheta, B. M., & Safran, R. J. (2018). Demographic inference in barn swallows using whole-genome data shows signal for bottleneck and subspecies differentiation during the Holocene. Molecular Ecology, 27(21), 4200–4212. http://doi.org/10.1111/mec.14854
- Sukumaran, J., & Knowles, L. L. (2018). Trait-Dependent Biogeography: (Re)Integrating Biology into Probabilistic Historical Biogeographical Models. Trends in Ecology & Evolution, 33(6), 390–398. http://doi.org/10.1016/j.tree.2018.03.010
- Sun, Y.-B., Fu, T.-T., Jin, J.-Q., Murphy, R. W., Hillis, D. M., Zhang, Y.-P., & Che, J. (2018). Species groups distributed across elevational gradients reveal convergent and continuous genetic adaptation to high elevations. Proceedings of the National Academy of Sciences, 115(45), E10634–E10641. http://doi.org/10.1073/pnas.1813593115
- Finally, a genomic adaptation study treats altitude as a gradient, rather than a dichotomy.
- Tang, Y., Winkler, J. A., Viña, A., Liu, J., Zhang, Y., Zhang, X., et al. (2018). Uncertainty of future projections of species distributions in mountainous regions. PloS One, 13(1), e0189496–23. http://doi.org/10.1371/journal.pone.0189496
- Thom, G., Amaral, F. R. D., Hickerson, M. J., Aleixo, A., Araujo-Silva, L. E., Ribas, C. C., et al. (2018). Phenotypic and Genetic Structure Support Gene Flow Generating Gene Tree Discordances in an Amazonian Floodplain Endemic Species. Systematic Biology, 67(4), 700–718. http://doi.org/10.1093/sysbio/syy004
- Torres, C. R., & Clarke, J. A. (2018). Nocturnal giants: evolution of the sensory ecology in elephant birds and other palaeognaths inferred from digital brain reconstructions. Proceedings of the Royal Society of London B: Biological Sciences, 285(1890), 20181540–8. http://doi.org/10.1098/rspb.2018.1540
- Troudet, J., Vignes-Lebbe, R., Grandcolas, P., & Legendre, F. (2018). The Increasing Disconnection of Primary Biodiversity Data from Specimens: How Does It Happen and How to Handle It? Systematic Biology, 67(6), 1110–1119. http://doi.org/10.1093/sysbio/syy044
- Uyeda, J. C., Zenil-Ferguson, R., & Pennell, M. W. (2017). Rethinking phylogenetic comparative methods, 1–44. Systematic Biology. http://doi.org/10.1101/222729
- Valencia, B. G., Bush, M. B., Coe, A. L., Orren, E., & Gosling, W. D. (2018). Polylepis woodland dynamics during the last 20,000 years. Journal of Biogeography, 45(5), 1019–1030. http://doi.org/10.1111/jbi.13209
- Van Doren, B. M., & Horton, K. G. (2018). A continental system for forecasting bird migration. Science, 361(6407), 1115–1118. http://doi.org/10.1126/science.aat7526
- Velotta, J. P., Ivy, C. M., Wolf, C. J., Scott, G. R., & Cheviron, Z. A. (2018). Maladaptive phenotypic plasticity in cardiac muscle growth is suppressed in high-altitude deer mice. Evolution, 72(12), 2712–2727. http://doi.org/10.1111/evo.13626
- Vickrey, A., Bruders, R., Kronenberg, Z., Mackey, E., Bohlender, R. J., Maclary, E., et al. (2018). Protein-coding variation and introgression of regulatory alleles drive plumage pattern diversity in the rock pigeon eLife. https://elifesciences.org/articles/34803
- Videvall, E. (2018). Plasmodium parasites of birds have the most AT-rich genes of eukaryotes. Microbial Genomics, 4(2), 907–9. http://doi.org/10.1099/mgen.0.000150
- Wen, Z., Wu, Y., Cheng, J., Cai, T., Du, Y., Ge, D., et al. (n.d.). Abundance of small mammals correlates with their elevational range sizes and elevational distributions in the subtropics. Ecography http://doi.org/10.1111/ecog
- Xu, B., Sun, G., Wang, X., Lu, J., Wang, I. J., & Wang, Z. (2017). Population genetic structure is shaped by historical, geographic, and environmental factors in the leguminous shrub Caragana microphylla on the Inner Mongolia Plateau of China. BMC Plant Biology http://doi.org/10.1186/s12870-017-1147-7
- Zenzal, T. J., Jr, Moore, F. R., Diehl, R. H., Ward, M. P., & Deppe, J. L. (2018). Migratory hummingbirds make their own rules: the decision to resume migration along a barrier. Animal Behaviour, 137, 215–224. http://doi.org/10.1016/j.anbehav.2018.01.019
- A notable advance in the application of micro-tracking devices to small birds (0.28 g transmitter glued to a 4-g bird), successful for short-distance tracking to understand local movements and departure of fall-migrant Ruby-throated Hummingbirds on the Alabama Gulf Coast.






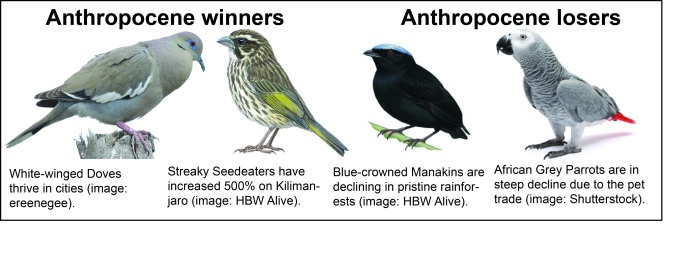 What can we do to help bird populations survive the Anthropocene?
What can we do to help bird populations survive the Anthropocene?